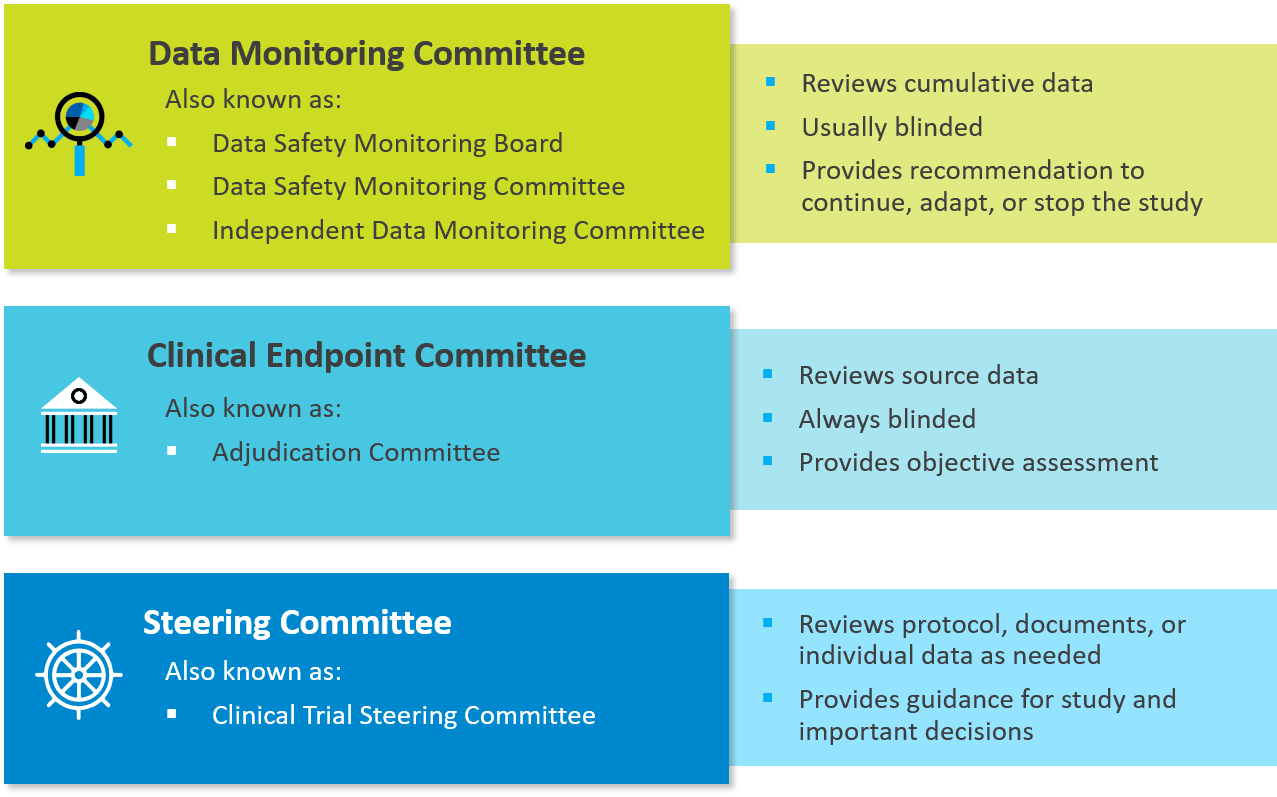
Guidelines for fracture healing assessments in clinical trials. Part I: Definitions and endpoint committees - Injury
Inadequate planning and reporting of adjudication committees in clinical trials: recommendation proposal
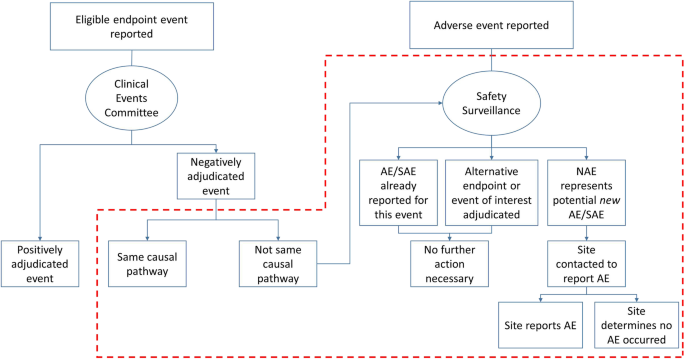
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text

Challenging Issues in Clinical Trial Design: Part 4 of a 4-Part Series on Statistics for Clinical Trials - ScienceDirect
Inadequate planning and reporting of adjudication committees in clinical trials: Recommendation proposal

Rationale and design of a prospective substudy of clinical endpoint adjudication processes within an investigator-reported randomised controlled trial in patients with coronary artery disease: the GLOBAL LEADERS Adjudication Sub-StudY (GLASSY) | BMJ
Subjective endpoints in clinical trials: the case for blinded independent central review - Document - Gale Academic OneFile

2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee
Clinical Trials: Minimising source data queries to streamline endpoint adjudication in a large multi-national trial

Development of Novel, Value-Based, Digital Endpoints for Clinical Trials: A Structured Approach Toward Fit-for-Purpose Validation | Pharmacological Reviews