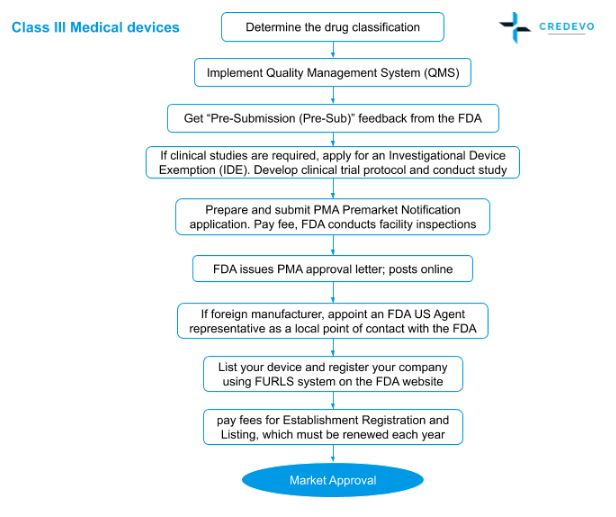
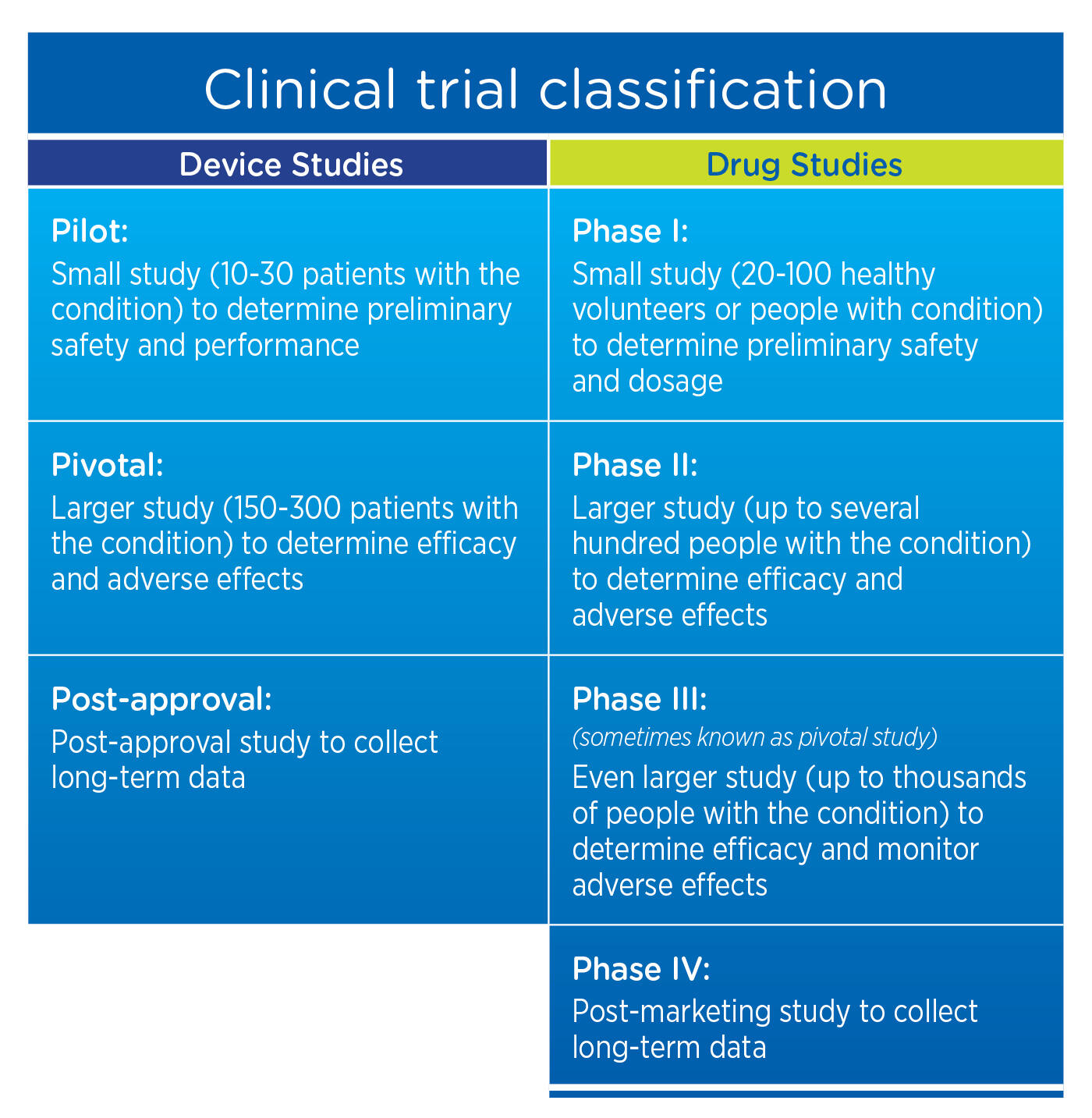
Guidance and Procedures: Use of Devices in Clinical Research and Brief Overview Investigational Device Exemption Applications (
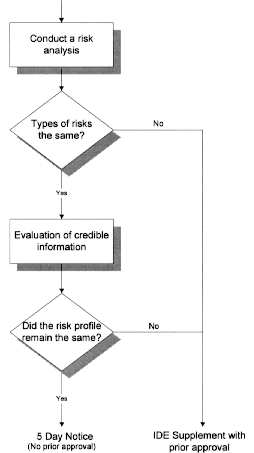
Changes or Modifications During the Conduct of a Clinical Investigation; Final Guidance for Industry and CDRH Staff | FDA
FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare and Medicaid Services (C
Live Case Presentations During Investigational Device Exemption (IDE) Clinical Trials - Guidance for Institutional Review Boards

Updated FDA Guidance Helps Device Study Sponsors Better Anticipate Coverage for Investigational Devices | Advisories | Arnold & Porter
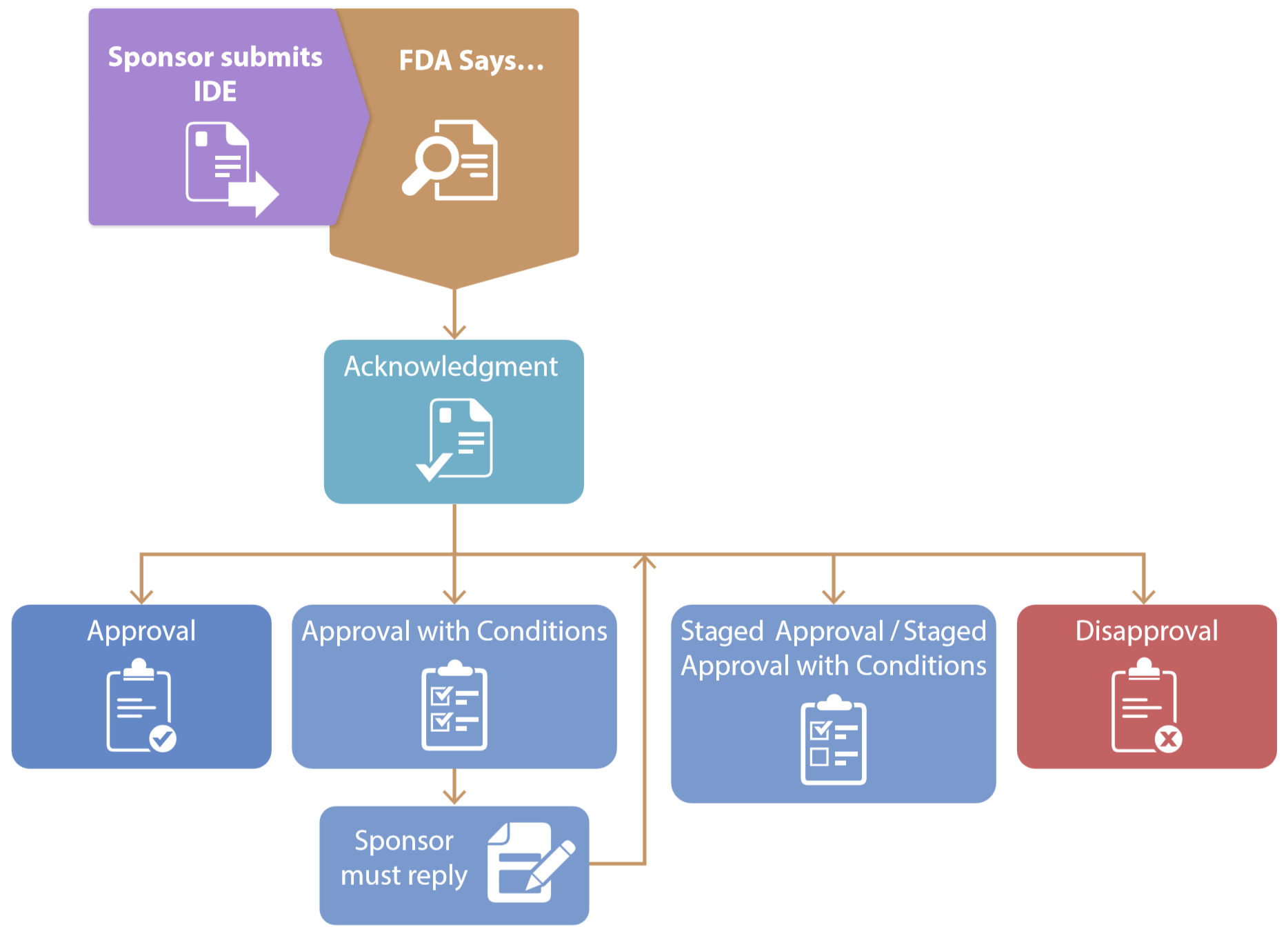
FDA Decisions for Investigational Device Exemption Clinical Investigations - Guidance for Sponsors, Clinical Investigators, Inst